New clinical trials unit will boost research at Clatterbridge
Posted 5th May 2022

Research capability at The Clatterbridge Cancer Centre NHS Foundation Trust is expanding with the creation of an Early Phase Trials Unit.
The Clinical Trials team at Clatterbridge Cancer Centre – Liverpool now has four in-patient beds, which will allow the Trust to increase experimental medicine and world-class research for cancer patients across the region.
Pictured right: the Early Phase Trails Unit Team with, centre from left, Demi-Leigh Tyrrell, Deputy Manager of Ward; Michelle Moffitt, Matron for Research and Innovation and Consultant Medical Oncologist Dr Anna Olsson-Brown.
Previously, in-patients on clinical trials were located in different areas of the hospital, but treatment and care can now be provided efficiently and effectively in the new unit where training, education and support can be further enhanced.
Consultant Medical Oncologist Dr Anna Olsson-Brown has been pivotal in setting up this dedicated space. She said: “We are delighted that the Early Phase Trials Unit has been created as this will greatly increase our ability to host early phase clinical trials at Clatterbridge Cancer Centre, widening trial opportunities for our patients and furthering research globally.
“The Clinical Trials team is excited to share the wonderful facilities with participants of our studies – the bedrooms for our inpatients are really second to none. It is important that patients who are participating in clinical trials feel safe and secure and a dedicated space really helps us to achieve that, allowing us to further our research and hopefully to create better treatments and care.
“I would like to thank everyone across the Trust who has supported us in making this happen.”
Clatterbridge consultant Prof Daniel Palmer is Clinical Director of the CRUK/NIHR Liverpool Experimental Cancer Medicine Centre (ECMC) and Lead for the Liverpool Clinical Research Facility (CRF) at CCC. He said: “We expect the number of early phase clinical trials to increase at Clatterbridge thanks to the successful national funding bid for the CRF. This funding in combination with the new unit will ensure we have the staffing infrastructure and state-of-the-art facilities to meet this demand.
“This dedicated area will also help us to further enhance the training and education we are able to provide to our workforce and handle more complex clinical trials more effectively and efficiently.”
Some treatments given on clinical trials, for example immunotherapy, must be administered in a contained environment and the unit has facilities for this, with an adjoining anteroom available where a consultant can observe and have a dialogue with the nurse administering the treatment to a patient, if required.
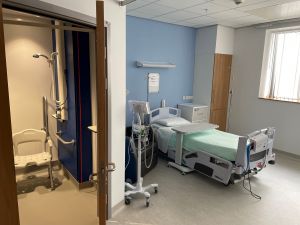

The four beds are in rooms 25 to 28 on Ward 4 of the hospital and are ring-fenced for research. However, beds not required by the Clinical Trials team at any time will be released back to the ward for use outside research.
Prof Christian Ottensmeier, Clatterbridge’s Director of Clinical Research, said: “The Early Phase Trials Unit is another example of how Clatterbridge is expanding its research capabilities to increase the number and complexity of our clinical trials, which are so crucial to extending our knowledge of treatments for cancer.
“It shows the Trust’s commitment to research and innovation at our new hospital in the Knowledge Quarter of Liverpool, where we have helped to create a growing community of people committed to furthering science and medicine that will improve outcomes for people with cancer across Cheshire and Merseyside.”
Pictured left: patient rooms on the Early Phase Trials Unit
